Synthesis of α-(aminoethyl)-α,β-enones via alkyne aza-Prins cyclization and their synthetic application to pyrrolidines†
Abstract
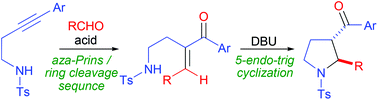
We developed a synthetic method for α-(aminoethyl)-α,β-enones from aryl-substituted homopropargyl sulfonamides and aldehydes, representing the first synthesis of conjugated enones via alkyne aza-Prins cyclization. These products could be converted into pyrrolidines by a formal 5-endo-trig cyclization.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...