A new asymmetric activation strategy for hydrazones as acyl anion equivalents in the bimetallic catalyzed carbonyl-ene reaction†
Abstract
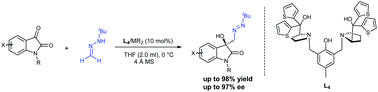
A new asymmetric activation strategy for hydrazones as acyl anion equivalents is developed in the bimetallic catalyzed carbonyl-ene reaction of isatins and hydrazones. Under mild conditions, optically active functionalized 3-hydroxy-2-oxindoles were furnished in up to 98% yield with up to 97% enantioselectivity. In this process, formaldehyde tert-butylhydrazone which is seldom employed in asymmetric carbonyl-ene reactions accelerated by a metallic catalyst can be activated well by a Brønsted base. A possible catalytic cycle is proposed.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...