Palladium(ii)-assisted activation of thioglycosides†
Abstract
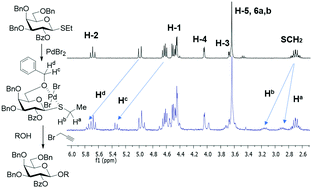
Described herein is the first example of glycosidation of thioglycosides in the presence of palladium(II) bromide. While the activation with PdBr2 alone was proven feasible, higher yields and cleaner reactions were achieved when these glycosylations were performed in the presence of propargyl bromide as an additive. Preliminary mechanistic studies suggest that propargyl bromide assists the reaction by creating an ionizing complex, which accelerates the leaving group departure. A variety of thioglycoside donors in reactions with different glycosyl acceptors were investigated to determine the initial scope of this new reaction. Selective and chemoselective activation of thioglycosides over other leaving groups has also been explored.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...