Asymmetric synthesis of 9-alkyl tetrahydroxanthenones via tandem asymmetric Michael/cyclization promoted by chiral phosphoric acid†
Abstract
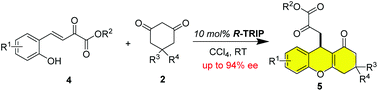
A tandem asymmetric Michael-addition/cyclization of cyclic 1,3-dicarbonyl compounds to β,γ-unsaturated α-ketoesters catalyzed by chiral phosphoric acid is presented. This protocol provides a facile approach for the construction of enantioenriched 9-alkyl tetrahydroxanthenones, an ubiquitous framework found in a number of natural products and pharmaceutical molecules, in high yields with good to high enantioselectivities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...