Pyrrole carboxamide introduction in the total synthesis of pyrrole–imidazole alkaloids
Abstract
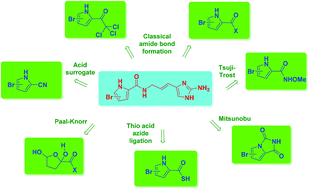
In this review various strategies for the incorporation of the signature pyrrole carboxamide moiety in the total syntheses of pyrrole–imidazole alkaloids (PIA) are discussed. These so-called oroidin alkaloids have a broad range of biological activities and display interesting skeletal diversity and complexity. These alkaloids are sponge-derived secondary metabolites and thus far more than 200 members of the PIA family have been isolated over the past few decades. Methods range from classical amide bond forming processes to non-traditional bond formation including the de novo synthesis of the pyrrole itself.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...