A practicable synthesis of 2,3-disubstituted 1,4-dioxanes bearing a carbonyl functionality from α,β-unsaturated ketones using the Williamson strategy†
Abstract
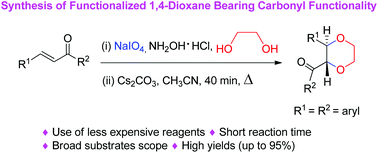
We have observed that a reagent combination of NaIO4 and NH2OH·HCl reacts with α,β-unsaturated ketones followed by the nucleophile ethylene glycol allowing the synthesis of 2,3-disubstituted 1,4-dioxanes using cesium carbonate as a base under Williamson ether synthesis. This reaction is useful for the synthesis of functionalized 1,4-dioxane having a carbonyl functionality. A variety of 2,3-disubstituted 1,4-dioxanes have been synthesized using these reaction conditions. A probable reaction mechanism has also been proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...