Structural and mechanical characteristics of exosomes from osteosarcoma cells explored by 3D-atomic force microscopy†
Abstract
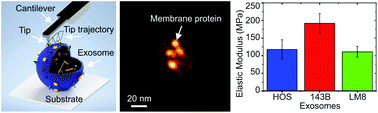
Exosomes have recently gained interest as mediators of cell-to-cell communication and as potential biomarkers for cancer and other diseases. They also have potential as nanocarriers for drug delivery systems. Therefore, detailed structural, molecular, and biomechanical characterization of exosomes is of great importance for developing methods to detect and identify the changes associated with the presence of cancer and other diseases. Here, we employed three-dimensional atomic force microscopy (3D-AFM) to reveal the structural and nanomechanical properties of exosomes at high spatial resolution in physiologically relevant conditions. The substructural details of exosomes released from three different cell types were determined based on 3D-AFM force mapping. The resulting analysis revealed the presence of distinct local domains bulging out from the exosome surfaces, which were associated with the exosomal membrane proteins present on the outer surface. The nanomechanical properties of individual exosomes were determined from the 3D-force maps. We found a considerably high elastic modulus, ranging from 50 to 350 MPa, as compared to that obtained for synthetic liposomes. Moreover, malignancy-dependent changes in the exosome mechanical properties were revealed by comparing metastatic and nonmetastatic tumor cell-derived exosomes. We found a clear difference in their Young's modulus values, suggesting differences in their protein profiles and other exosomal contents. Exosomes derived from a highly aggressive and metastatic k-ras-activated human osteosarcoma (OS) cell line (143B) showed a higher Young's modulus than that derived from a nonaggressive and nonmetastatic k-ras-wildtype human OS cell line (HOS). The increased elastic modulus of the 143B cell-derived exosomes was ascribed to the presence of abundant specific proteins responsible for elastic fiber formation as determined by mass spectroscopy and confirmed by western blotting and ELISA. Therefore, we conclude that exosomes derived from metastatic tumor cells carry an exclusive protein content that differs from their nonmetastatic counterparts, and thus they exhibit different mechanical characteristics. Discrimination between metastatic and nonmetastatic malignant cell-derived exosomes would be of great importance for studying exosome biological functions and using them as diagnostic biomarkers for various tumor types. Our findings further suggest that metastatic tumor cells release exosomes that express increased levels of elastic fiber-associated proteins to preserve their softness.


 Please wait while we load your content...
Please wait while we load your content...