Beyond peptide bond formation: the versatile role of condensation domains in natural product biosynthesis
Abstract
Covering: up to the end of 2020
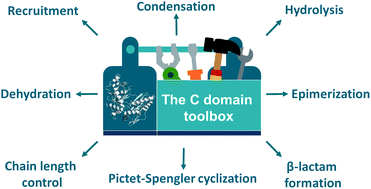
Nonribosomal peptide synthetases are remarkable molecular machines that produce a wide range of structurally complex peptide natural products with important applications in medicine and agriculture. Condensation domains play a central role in these biosynthetic pathways by catalysing amide bond formation between various aminoacyl substrates. In recent years, however, it has become increasingly clear that the catalytic repertoire of C domains extends far beyond conventional peptide bond formation. C domains have been shown to perform highly diverse functions during nonribosomal peptide assembly, such as β-lactam formation, dehydration, hydrolysis, chain length control, cycloaddition, Pictet–Spengler cyclization, Dieckmann condensation and recruitment of auxiliary enzymes. In this review, a comprehensive overview of the multifaceted role of C domains in the biosynthesis of specialized metabolites in bacteria and fungi is presented. Different perspectives are also offered on how the exceptional functional versatility of C domains may be exploited for bioengineering approaches to expand the chemical diversity of nonribosomal peptides and other natural products.


 Please wait while we load your content...
Please wait while we load your content...