Heptose-containing bacterial natural products: structures, bioactivities, and biosyntheses
Abstract
Covering: up to 2020
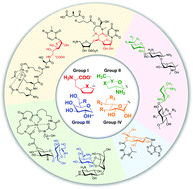
Glycosylated natural products hold great potential as drugs for the treatment of human and animal diseases. Heptoses, known as seven-carbon-chain-containing sugars, are a group of saccharides that are rarely observed in natural products. Based on the structures of the heptoses, the heptose-containing natural products can be divided into four groups, characterized by heptofuranose, highly-reduced heptopyranose, D-heptopyranose, and L-heptopyranose. Many of them possess remarkable biological properties, including antibacterial, antifungal, antitumor, and pain relief activities, thereby attracting great interest in biosynthesis and chemical synthesis studies to understand their construction mechanisms and structure–activity relationships. In this review, we summarize the structural properties, biological activities, and recent progress in the biosynthesis of bacterial natural products featuring seven-carbon-chain-containing sugars. The biosynthetic origins of the heptose moieties are emphasized.


 Please wait while we load your content...
Please wait while we load your content...