A theoretical study of the hydroboration of α,β-unsaturated carbonyl compounds catalyzed by a metal-free complex and subsequent C–C coupling with acetonitrile†
Abstract
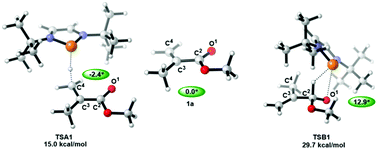
Herein, the density functional theory (DFT) method was employed to investigate the reaction mechanism of the selective hydroboration of α,β-unsaturated carbonyl compounds catalyzed by the metal-free complex 1,3,2-diazaphospholene (DAP). The reaction mechanism consisted of two parts: one being the hydroboration of α,β-unsaturated carbonyl compounds and the other C–C coupling of the hydroboration product with CH3CN. 1,4- and 1,2-hydroboration pathways were considered for the DAP-catalyzed hydroboration of the α,β-unsaturated carbonyl compound. The free energy barrier of the rate-determining step (RDS, the first hydrogen transfer step) of the 1,4-hydroboration of the α,β-unsaturated ester was calculated to be 15.0 kcal mol−1, and that of the RDS (the first hydrogen transfer step) of the 1,2-hydroboration pathway was 29.7 kcal mol−1. These results indicated the greater favorability of the 1,4-hydroboration pathway than of the 1,2-hydroboration pathway for the hydroboration of α,β-unsaturated esters. This difference might be due to a greater extent of degradation of the conjugated π system in the hydrogen transfer transition state of the 1,2-hydroboration pathway than in that of the 1,4-hydroboration pathway. Meanwhile, the 1,2- and 1,4-hydroboration pathways without DAP catalysts were also theoretically investigated, and this investigation revealed the ability of DAP to not only reduce the free energy barrier of hydroboration but also to change the chemoselectivity. In addition, an analysis of NAO energy, Hirshfeld charge, and NBO charge was performed to investigate the effects of substrate substituents on chemical reactivity; these indicators of the key atoms in the first hydrogen transfer steps in the 1,2- and 1,4-hydroboration pathways were indicated to have good linear relationships with the corresponding energy barriers. This work could provide much-needed theoretical guidance for designing better metal-free catalysts for selective hydroboration reactions.


 Please wait while we load your content...
Please wait while we load your content...