Symmetry driven: the synthesis of co-substituent octasilsesquioxanes†
Abstract
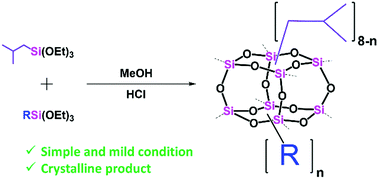
Octasilsesquioxane cages with co-substituent characteristics were synthesized through polyhydrolytic–condensation reactions involving two mixed monomers, namely i-butyl(triethoxysilane) and organotrialkoxysilanes (e.g., phenyl, vinyl, ethyl, propyl, and 3-chloropropyl groups). Only mild and simple methods were required to obtain crystalline products as all products consisted octameric silsesquioxane (T8) cubes with normal distribution between i-butyl and other substituents. The formation was confirmed by NMR and ESI-MS spectra. The i-butyl group on the silane monomer was not only a controller to yield cage-like structural products with mixed substituents, but also their highly symmetrical Oh in the cubic Si–O core, which drives the product separation through crystallization. The crystalline structure of the products was validated by the correlation between the endothermic peak at the melting temperature (Tm) from the DSC analysis and sharp peaks observed in the XRD diffraction patterns.


 Please wait while we load your content...
Please wait while we load your content...