Photophysical properties of 4-(5-methylthiophen-2-yl)pyridinium–cyclic enolate betaine dyes tuned by control of twisted intramolecular transfer†
Abstract
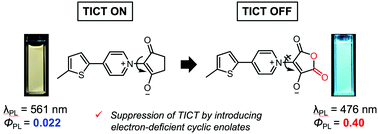
In this paper, thienylpyridinium–cyclic enolate betaine (TPB) dyes were reported as unique skeletons of fluorescent donor–acceptor type molecules. TPB derivatives with five-membered cyclic enolates are coplanar because of the intramolecular hydrogen bond formation between the carbonyl oxygens of the cyclic enolate and the α-hydrogens of the pyridinium ring. However, a six-membered cyclic enolate leads to a pre-twisted structure due to the steric hindrance. TPB derivatives with alkylene-bridged cyclic enolates exhibited obvious fluorescence quenching through the twisted intramolecular charge transfer (TICT) process in solution. In contrast, electron-deficient cyclic enolates suppressed the TICT process, and thus relatively intense emission was observed. This report also discusses deactivation processes in the excited state of each TPB derivative by theoretical calculations to support the experimental results. These observations present a rational design strategy to control the TICT of TPB derivatives.


 Please wait while we load your content...
Please wait while we load your content...