Cascaded filter deterministic lateral displacement microchips for isolation and molecular analysis of circulating tumor cells and fusion cells†
Abstract
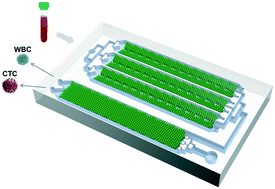
Precise isolation and analysis of circulating tumor cells (CTCs) from blood samples offer considerable potential for cancer research and personalized treatment. Currently, available CTC isolation approaches remain challenging in the quest for simple strategies to achieve cell isolation with both high separation efficiency and high purity, which limits the use of captured CTCs for downstream analyses. Here, we present a filter deterministic lateral displacement concept to achieve one-step and label-free CTC isolation with high throughput. Unlike conventional deterministic lateral displacement (DLD) devices, the proposed method uses a hydrodynamic cell sorting design by incorporating a filtration concept into a DLD structure, and enables high-throughput and clog-free isolation by a cascaded microfluidic design. The cascaded filter-DLD (CFD) design demonstrated enhanced performance for size-based cell separation, and achieved high separation efficiency (>96%), high cell purity (WBC removal rate 99.995%), high cell viability (>98%) and high processing rate (1 mL min−1). Samples from lung cancer patients were analyzed using the CFD-Chip, CTCs and tumor cell–leukocyte fusion cells were efficiently collected, and changes in CTC levels were used for treatment response monitoring. The CFD-Chip platform isolated CTCs with good viability, enabling direct downstream analysis with single-cell RNA sequencing. Transcriptome analysis of enriched CTCs identified new subtypes of CTCs such as tumor cell–leukocyte fusion cells, providing insights into cancer diagnostics and therapeutics.


 Please wait while we load your content...
Please wait while we load your content...