Identification and expression of a CHMO from the Pseudomonas aeruginosa strain Pa1242: application to the bioconversion of Cyrene™ into a key precursor (S)-γ-hydroxymethyl-butyrolactone†
Abstract
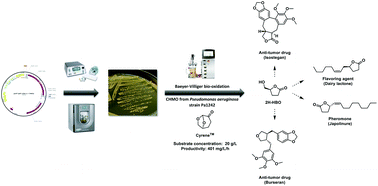
A sequence from the complete genome of the Pseudomonas aeruginosa strain Pa1242 was identified to code for a potentially new cyclohexanone monooxygenase (CHMO). The latter was discovered using the Basic Alignment Search Tool (BLAST) with the DNA sequence of the CHMO from Acinetobacter sp. NCIB 9871 as the parent sequence considering that this enzyme family maintains a relatively high query cover (>95%) and identity percentage (>50%) among many different microorganisms. This was confirmed by the bioconversion of the cellulose-based green solvent Cyrene™ into a key precursor (S)-γ-hydroxymethyl-butyrolactone (2H-HBO) using a new strain of E. coli BL21(DE3) containing a plasmid designed for the overexpression of the unprecedented CHMO (pLM7). Besides confirming the CHMO activity of this novel sequence, this bioconversion allows the obtention of 2H-HBO while maintaining the naturalness of the process. The optimal culture conditions were assessed through a Design of Experiments (DoE), and the control of the pH and O2 level allowed to reach a working concentration of 20 g L−1 of Cyrene™ with total conversion and a productivity rate of 401 ± 36 mg L−1 h−1.


 Please wait while we load your content...
Please wait while we load your content...