Room temperature iron catalyzed transfer hydrogenation using n-butanol and poly(methylhydrosiloxane)†
Abstract
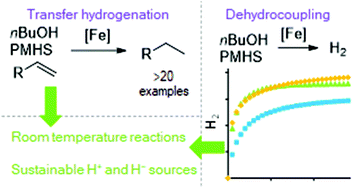
Reduction of carbon–carbon double bonds is reported using a three-coordinate iron(II) β-diketiminate pre-catalyst. The reaction is believed to proceed via a formal transfer hydrogenation using poly(methylhydrosiloxane), PMHS, as the hydride donor and a bio-alcohol as the proton source. The reaction proceeds well using n-butanol and ethanol, with n-butanol being used for substrate scoping studies. Allyl arene substrates, styrenes and aliphatic substrates all undergo reduction at room temperature. Unfortunately, clean transfer of a deuterium atom using D-alcohol does not take place, indicating a complex catalytic mechanism. However, changing the deuterium source to D-aniline gives close to complete regioselectivity for mono-deuteration of the terminal position of the double bond. Finally, we demonstrate that efficient dehydrocoupling of alcohol and PMHS can be undertaken using the same pre-catalyst, giving high yields of H2 within 30 minutes at room temperature.


 Please wait while we load your content...
Please wait while we load your content...