Highly enantioselective immobilized prolinamide-catalyzed aldol reactions in continuous-flow systems: effect of water on the catalyst lifetime and application in the synthesis of a chiral fenpentadiol analogue†
Abstract
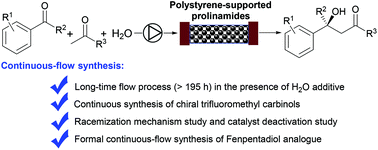
Catalytic enantioselective aldol reactions of trifluoroacetophenones with ketones under continuous-flow conditions have been developed for the first time by using polystyrene-supported prolinamides. The robustness of the flow system was demonstrated by the continuous synthesis of a variety of trifluoromethyl carbinols in high yields with high enantioselectivities. The unusually long lifetimes (>195 h) of this flow process were achieved by facilitating H2O-promoted hydrolysis of iminium intermediates on the polymer. Mechanistic study revealed a racemization phenomenon and an inherent reversible property of the aldol reactions in a conventional batch system, both of which were suppressed under continuous-flow conditions. The synthetic utility of this flow process was further demonstrated by the formal continuous-flow synthesis of a chiral fenpentadiol analogue.


 Please wait while we load your content...
Please wait while we load your content...