Choline chloride-promoted efficient solvent-free hydrogenation of biomass-derived levulinic acid to γ-valerolactone over Ru/C†
Abstract
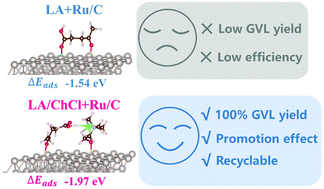
It is highly attractive to produce γ-valerolactone (GVL), an all-purpose biomass-derived platform molecule, by the solvent-free hydrogenation of levulinic acid (LA), but the intense adsorption of the carboxylic group over the catalyst will block the active sites of the catalyst. In this study, the established hydrogen bond between LA and choline chloride (ChCl) suppressed the adsorption of the carboxylic group but enhanced the adsorption of the acyl group of LA over Ru/C, which greatly improved the hydrogenation of the acyl group in LA to afford quantitative GVL yield under solvent-free conditions.


 Please wait while we load your content...
Please wait while we load your content...