Direct Diels–Alder reactions of furfural derivatives with maleimides†
Abstract
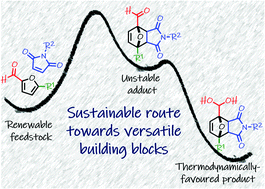
The Diels–Alder (DA) reaction of furans is a versatile tool in synthetic organic chemistry and in the production of sustainable building blocks and smart materials. Numerous experimental and theoretical investigations suggest that the diene scope is effectively limited to electron-rich furans, which excludes the most abundant and readily accessible renewable derivatives: furfural and its 5-hydroxymethyl homologue. Herein we show for the first time that electron-poor 2-formylfurans can also directly engage in Diels–Alder couplings. The key to success is the use of aqueous medium, which supplies an additional thermodynamic driving force by coupling the unfavorable DA equilibrium to the exergonic hydration of the carbonyl functionality in the adducts to form geminal diols. This finding enables the direct access to various novel DA adducts derived from renewable furfurals and maleimides, via a mild, simple and environmentally-friendly synthetic protocol.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...