Solar-driven electrochemical synthesis of ammonia using nitrate with 11% solar-to-fuel efficiency at ambient conditions†
Abstract
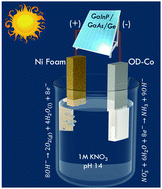
Ammonia is an essential commodity chemical used in the manufacture of fertilizers, pharmaceuticals, ammunition, and plastics, and is a promising alternative fuel source and carrier. Today most ammonia is manufactured by the century-old Haber–Bosch process, which accounts for 1–2% of worldwide energy production and a substantial fraction of global greenhouse gas emissions. Solar-driven electrochemical synthesis of ammonia using nitrates presents a sustainable pathway to produce renewable fuels utilizing wastewater. Previous efforts in solar-driven electrosynthesis of ammonia have been seriously affected by lower specific activity (<10 mA cm−2) of electrochemical nitrate reduction reaction (NiRR) and thereby lower solar-to-fuel (STF) efficiency (<1%). Here, we show oxide-derived Co as an efficient NiRR catalyst with the highest specific activity (∼14.56 mA cm−2 at −0.8 V vs. RHE) and selectivity. The oxide-derived Co offers a maximum faradaic efficiency of 92.37 ± 6.7% and ammonia current density of 565.26 mA cm−2 at −0.8 V vs. RHE. Integrating this catalyst in a PV-electrolyzer cell yields an unprecedented STF efficiency of 11% for ammonia, which is an order of magnitude higher than state-of-the-art systems.


 Please wait while we load your content...
Please wait while we load your content...