High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 hours†
Abstract
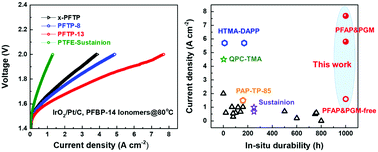
Low-cost anion exchange membrane (AEM) water electrolyzers (AEMWEs) are a new technology for the production of high-purity hydrogen; however, their current density and durability are far lower than those of proton exchange membrane water electrolyzers (PEMWEs). Here, we report poly(fluorenyl-co-aryl piperidinium) (PFAP)-based anhydrous cathode AEMWEs that exceed the state-of-the-art PEMWEs with respect to current density. In addition to a rational electrode design, PFAP-based AEMs with a high water diffusivity and ion conductivity are crucial for high-performance AEMWEs. Using platinum-group-metal (PGM) catalysts, the present AEMWEs achieved a new record current density of 7.68 A cm−2 at 2.0 V with a 1 M KOH anode, which surpasses that of state-of-the-art PEMWEs (6 A cm−2 at 2.0 V). PGM-free AEMWEs displayed an excellent current density of 1.62 A cm−2 at 2.0 V. Importantly, PGM and PGM-free AEMWEs operated stably under a 0.5 A cm−2 current density at 60 °C for more than 1000 h. This work sheds light on current high-performance AEMWEs.


 Please wait while we load your content...
Please wait while we load your content...