Coupling of CO2 and epoxides catalysed by novel N-fused mesoionic carbene complexes of nickel(ii)†
Abstract
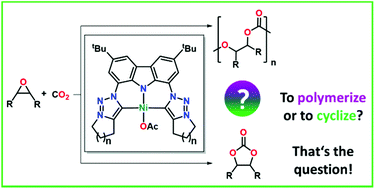
We report the syntheses of two rigid mesoionic carbene (MIC) ligands with a carbazole backbone via an intramolecular Finkelstein–cyclisation cascade and investigate their coordination behavior towards nickel(II) acetate. Despite the nickel(II) carbene complexes 4a,b showing only minor differences in their chemical composition, they display curious differences in their chemical properties, e.g. solubility. Furthermore, the potential of these novel MIC complexes in the coupling of carbon dioxide and epoxides as well as the differences in reactivity compared to classical NHC-derived complexes are evaluated.


 Please wait while we load your content...
Please wait while we load your content...