A cyanide-bridged Fe–Co pearl-chain-like single-chain magnet containing 4-coordinate cobalt(ii) ions†
Abstract
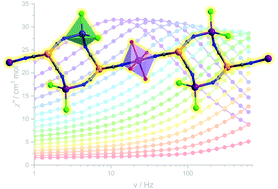
Treatment of CoCl2·6H2O and tris(pyrazolyl-1-yl)borate tricyanoiron(III) anions at an elevated temperature (55 °C) afforded two less-common pearl-chain-like compounds, {[(TpR)Fe(CN)3CoCl2]2Co(DMF)4}·nDMF (1, TpR = Tp4-Me = hydridotris(4-methylpyrazol-1-yl)borate, n = 1 and 2, TpR = Tp*Me = hydridotris(3,4,5-trimethylpyrazol-1-yl)borate, n = 4.5), in which the 4-coordinate Co(II) ions and [(TpR)FeIII(CN)3]− units are alternately bridged by cyanide groups into squares, which are further linked with the 6-coordinate Co(II) ions into an infinite chain. Interestingly, the magnetic study revealed that 1 exhibits a typical single-chain magnet behaviour with an effective energy barrier of 28.0 K, while surprisingly no Glauber dynamics was observed for 2 despite their very similar structures. The variations of the local coordination environments of the cobalt ions and the cyanide linkages were evidenced, and they may account for the significant difference in their magnetic properties related to the global magnetic anisotropy and magnetic exchange of the chain.


 Please wait while we load your content...
Please wait while we load your content...