Solid-state 11B NMR studies of coinage metal complexes containing a phosphine substituted diboraanthracene ligand†
Abstract
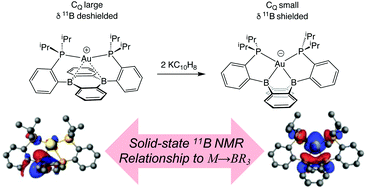
Transition metal interactions with Lewis acids (M → Z linkages) are fundamentally interesting and practically important. The most common Z-type ligands contain boron, which contains an NMR active 11B nucleus. We measured solid-state 11B{1H} NMR spectra of copper, silver, and gold complexes containing a phosphine substituted 9,10-diboraanthracene ligand (B2P2) that contain planar boron centers and weak M → BR3 linkages ([(B2P2)M][BArF4] (M = Cu (1), Ag (2), Au (3)) characterized by large quadrupolar coupling (CQ) values (4.4–4.7 MHz) and large span (Ω) values (93–139 ppm). However, the solid-state 11B{1H} NMR spectrum of K[Au(B2P2)]− (4), which contains tetrahedral borons, is narrow and characterized by small CQ and Ω values. DFT analysis of 1–4 shows that CQ and Ω are expected to be large for planar boron environments and small for tetrahedral boron, and that the presence of a M → BR3 linkage relates to the reduction in CQ and 11B NMR shielding properties. Thus solid-state 11B NMR spectroscopy contains valuable information about M → BR3 linkages in complexes containing the B2P2 ligand.


 Please wait while we load your content...
Please wait while we load your content...