Enhancing the oxide-ionic conductivity of Ba3Mo1+xNb1−2xGexO8.5 at intermediate temperatures: the effect of site-selective Ge4+-substitution†
Abstract
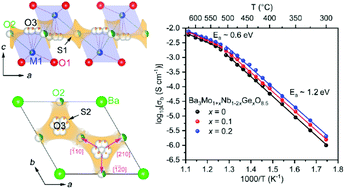
Significant oxide-ionic conductivity has been recently reported for a family of cation-deficient hexagonal perovskite derivatives Ba3M2O8.5 (M = Mo/W6+ and Nb5+/V5+). Herein, strong 4-fold coordination geometry preferring Ge4+ ions are doped into Ba3Mo1+xNb1−2xGexO8.5 to manipulate the oxygen distribution within palmierite-like layers for the enhancement of oxide-ionic conductivity. Rietveld refinement of the neutron diffraction data of Ba3Mo1.2Nb0.6Ge0.2O8.5 reveals that Ge4+-ions are selectively incorporated into the palmierite-like layers, owing to their strong 4-fold coordination environment preference. Such a site-selective doping behavior leads to an increase in the occupation proportion of the O3 site and a concomitant decrease in the occupancy factor for O2. Ionic conduction measurements show that the bulk conductivity of Ba3Mo1.2Nb0.6Ge0.2O8.5 is about twice higher than that of the parent compound at intermediate temperatures (300–500 °C). Furthermore, bond-valence site energy (BVSE) landscape analysis reveals that the oxygen ionic conduction of Ba3Mo1+xNb1−2xGexO8.5 is dominated by the two-dimensional pathways along the palmierite-like layers, despite the three-dimensional (3D) oxygen diffusion pathways being present in the hybrid structure, which strongly confirms that the enhancement in ionic conductivity at intermediate temperatures is attributed to the site-selective Ge4+-substitution-induced redistribution of oxygen ions within the palmierite-like layers.


 Please wait while we load your content...
Please wait while we load your content...