Incorporation of iodine into uranium oxyhydroxide phases†
Abstract
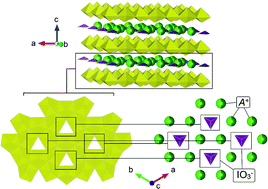
Herein, we have synthesised a novel uranium oxyhydroxide (UOH) phase, Rb2K2[(UO2)6O4(OH)6]·(IO3)2, under hydrothermal conditions which intercalates IO3−via a hybrid salt-inclusion and host–guest mechanism. The mechanism is based on favorable intermolecular bonding between disordered Rb+/K+ and IO3− ions and hydroxyl and layer void positions respectively. To examine whether the intercalation may occur ubiquitously for UOH phases, the known UOH mineral phases metaschoepite ([(UO2)8O2(OH)12]·12H2O), compreignacite (K2[(UO2)6O4(OH)6]·7H2O) and also related β-UO2(OH)2 were synthesised and exposed to aqueous I− and IO3− for 1 month statically at RT and 60 °C in air and the solid analysed using laser ablation inductively coupled plasma mass spectroscopy. Measurements indicate intercalation can occur homogeneously, but the affinity is dependent upon the structure of the UOH phases and temperature, where higher temperatures and when the interlayer space is free of initial moieties are favoured. It was also found that after repeated washing of the UOH samples with DI water the intercalated iodine was retained. UOH phases are known to form during the oxidative corrosion of spent nuclear fuel during an accident scenario in the near field, this work suggests they may help retard the transport of radiolytic iodine into the environment during a long-term release event.


 Please wait while we load your content...
Please wait while we load your content...