An asymmetric cryptand for the site-specific coordination of 3d metals in multiple oxidation states†
Abstract
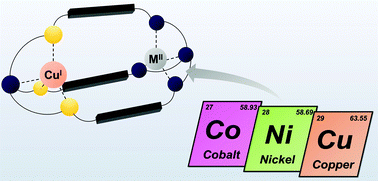
Bis-tren (tren = tris(2-aminoethyl)amine) azacryptands were previously studied profoundly for the coordination of two +II metals and subsequent binding of substrates within their cavity. Likewise, cryptates including metals in different oxidations states were reported with the rather unstable hexa-imine analogues but also revealed only little stability. In this work, we report the synthesis of an asymmetric hexa-amine cryptand analogue by selectively exchanging three of the secondary amines of one binding site with sulphur atoms. We show that the presence of two distinct binding sites allows for the selective formation of stable dinuclear complexes of metals with different oxidation numbers and present the formation of distinct CuICoII, CuINiII and CuICuIII cryptates.


 Please wait while we load your content...
Please wait while we load your content...