Chiroptical switching behavior of heteroleptic ruthenium complexes bearing acetylacetonato and tropolonato ligands†
Abstract
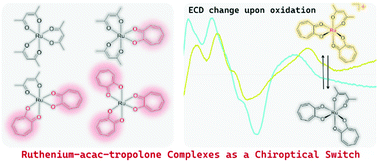
Four types of tris-chelate ruthenium complexes bearing acetylacetonato (acac) and tropolonato (trop) ligands were synthesized and optically resolved into Δ and Λ isomers: [Ru(acac)3] (Ru-0), [Ru(acac)2(trop)] (Ru-1), [Ru(acac)(trop)2] (Ru-2), and [Ru(trop)3] (Ru-3). Chiral HPLC chromatograms, electronic circular dichroism (ECD), and vibrational circular dichroism (VCD) of the four ruthenium complexes were systematically investigated. As a result, the absolute configurations of the newly prepared enantiomeric complexes Ru-2 and Ru-3 were determined. For the case of Ru-2, its absolute configuration was also confirmed by single crystal X-ray diffraction analysis. The ECD changes upon chemical oxidation were further investigated for the four complexes. An ECD change in enantiomeric Ru-1 was observed upon oxidation, but the oxidized species soon returned to the neutral state within a few minutes. Enantiomers of Ru-3 also showed explicit ECD changes upon oxidation. Further, the lifetime of the oxidized state was the longest among the four investigated complexes, whereas they racemized in solution at room temperature. In contrast, the enantiomers of heteroleptic complexes (Ru-1 and Ru-2) concurrently exhibited ECD changes, relatively long lifetime of the oxidized state, and nil or quite slow racemization behavior. The coexistence of acac and trop ligands was key to making the competing factors compatible in the resultant ruthenium complexes.


 Please wait while we load your content...
Please wait while we load your content...