Vapochromic behaviour of a nickel(ii)-quinonoid complex with dimensional changes between 1D and higher†
Abstract
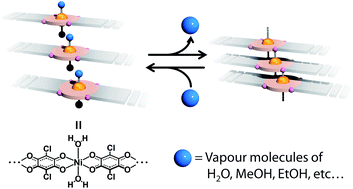
The nickel(II)–chloranilato complex {Ni(ca)(VM)2}n (H2ca = chloranilic acid, VM = coordinated vapour molecules, such as water) shows reversible vapochromism upon exposure to various vapours and subsequent drying by heating. In contrast to the Ni(II)-quinonoid complex, [Ni(HLMe)2] (H2LMe = 4-methylamino-6-methyliminio-3-oxocyclohexa-1,4-dien-1-olate), which was reported to exhibit vapochromic spin-state switching between high and low spin states, the chloranilato complex does not change its spin state even after the removal of coordinated vapour molecules. X-ray absorption fine structure (XAFS) analysis revealed that the six-coordinate geometry of {Ni(ca)(VM)2}n was maintained even after the removal of vapour molecules, in contrast to the [Ni(HLMe)2] complex. The unique vapochromism that follows the dimensional change between 1D and higher is influenced by the relatively weaker ligand field of the chloranilate ligand.


 Please wait while we load your content...
Please wait while we load your content...