Pentamethyl- and 1,2,4-tri(tert-butyl)cyclopentadienyl containing p-block complexes – differences and similarities†
Abstract
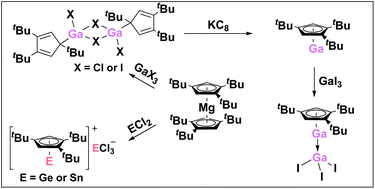
The sterically encumbered cyclopentadienyl ligand 1,2,4-(Me3C)3C5H2 (Cp′′′) was used to stabilize efficiently the main group metals of Al, Ga, In, Ge and Sn, respectively. The σ-bonded gallium compounds [η1-Cp′′′Ga(μ-X)X]2 (X = Cl, 2; X = I, 3) and indium compound [η1-Cp′′′In(μ-Br)nBu]2 (7) exhibit dimers through halogen bridges. Reduction of 2 with 2 equivalents of KC8 leads almost to the same amount of η1-Cp′′′Ga(THF)Cl2 (4) and η5-Cp′′′Ga (5), respectively. The exception is compound 5, which is obtained by reducing 2 or 3 with 4 equivalents of KC8. Compound 5 as Lewis base reacts with GaI3 readily forming the Lewis acid–base adduct product η5-Cp′′′Ga → GaI3 (6). Moreover, compounds with the Cp′′′ ligand stabilize heavier low-valent group 14 elements for example [η5-Cp′′′EII]+[EIICl3]− (E = Ge 8, Sn 9), which are π-bonded ionic compounds that possess a low-valent cation and an anion. In the cation of [η5-Cp′′′EII]+, the Cp′′′ ligand adopts an η5-coordination mode with germanium and tin, respectively, which present half-sandwich complexes. While the EII fragment interacts with five π electrons from the Cp′′′ unit to generate an electron-octet arrangement at the respective element. All new reported structures are comparing well with the corresponding compounds containing the pentamethylcyclopentadienyl (Cp*) ligand.


 Please wait while we load your content...
Please wait while we load your content...