Evidence of protonation induced intra-molecular metal-to-metal charge transfer in a highly symmetric cyanido bridged {Fe2Ni2} molecular square†
Abstract
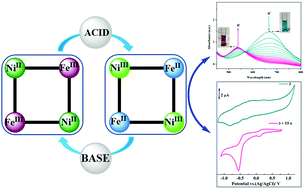
The possibility to induce intra-molecular metal-to-metal charge transfer in a cyanido bridged tetranuclear square shaped {Fe2Ni2} complex by employing protonation as an external stimulant is explored. Two cyanido bridged square shaped tetranuclear complexes, [{Ni(TPA)(μ2-NC)2Ni(CN)2}2]·4H2O (2) and [{Ni(TPA)(μ2-NC)2Fe(bbp)(CN)}2]·10H2O (3) [TPA = tris(3,5-dimethylpyrazol-1-ylmethyl)amine and H2bbp = bis(2-benzimidazolyl)pyridine], are synthesized and characterized. Low temperature magnetic measurements reveal that complex 3 has dominant ferromagnetic interactions between low-spin FeIII (S = 1/2) and high-spin NiII (S = 1) ions. UV-visible spectrophotometric measurements and electrochemical studies establish that reversible intra-molecular metal-to-metal electron transfer can be triggered in 3 upon the addition of either an acid or a base.


 Please wait while we load your content...
Please wait while we load your content...