Anion and solvent controlled growth of crystalline and amorphous zinc(ii) coordination polymers and a molecular complex†
Abstract
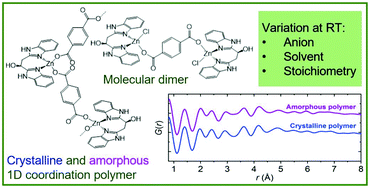
Controlled bottom-up synthesis of amorphous coordination polymers with tailored metal coordination is a research field in its infancy. In this study, synthesis control was achieved to selectively prepare one-dimensional (1D) crystalline and amorphous zinc(II)-based coordination polymers and a dimeric molecular compound, all with similar coordination geometry as evidenced by X-ray diffraction and total scattering studies. The compounds were obtained by bottom up self-assembly of Zn(II) with terephthalate (tph2−) as linker and the enantiopure chelating ligand S-(1,2)-bis(1H-benzimidazol-2-yl)ethanol (L). The solvent and the coordination ability of the precursor zinc salt anion control the crystalline products formed by slow diffusion at room temperature: perchlorate allows isolation of the phase pure crystalline 1D polymer {[Zn(tph)(L)]·H2O·3DMF}n (1·H2O·3DMF, DMF = N,N-dimethylformamide). In contrast, zinc chloride leads to the formation of either a mixture of polymeric 1·H2O·3DMF and a dimeric molecular species [Zn2Cl2(tph)(L)2]·4DMF (2·4DMF), or to the phase pure dimer 2·4DMF, depending on the Zn(II) : tphH2 stoichiometry. A modified synthesis using zinc nitrate and fast precipitation by base addition results in an amorphous analogue of the 1D polymer (3). Chains of 1·H2O·3DMF pack into a non-porous crystalline material with a surface area of just 6 m2 g−1, while the outer surface area of amorphous polymer 3 is a factor of eight larger. Hence, the amorphous compound provides larger metal site accessibility for potential surface chemical reactions, while maintaining the coordination geometry of the metal sites. The temperature response of crystalline polymer 1·H2O·3DMF was studied using multi-temperature single crystal X-ray diffraction (100–300 K). The a = b axes display normal positive thermal expansion, while the c axis remains constant with increasing temperature due to partial relaxation of the terephthalate linkers and slightly changed geometry within the individual polymer chains.


 Please wait while we load your content...
Please wait while we load your content...