Unravelling the dissolution mechanism of polyphosphate glasses by 31P NMR spectroscopy: ligand competition and reactivity of intermediate complexes
Abstract
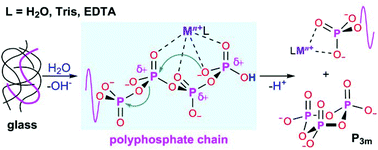
Phosphate glass dissolution can be tailored via compositional and subsequent structural changes, which is of interest for biomedical applications such as therapeutic ion delivery. Here, solid-state 31P nuclear magnetic resonance characterisation of 45P2O5 − xCaO − (55 − x)Na2O glasses was correlated with dissolution studies using time-dependent liquid 31P NMR spectroscopy and quantitative chemical analysis. Glasses dissolved congruently in aqueous media, and the first dissolution stage was the hydration of phosphate chains. In deionised water and Tris buffer (pH0 7.4 or 7.9), trimetaphosphate rings and orthophosphates were the predominant species in solution, indicating relatively fast degradation. By contrast, long phosphate chains were identified in EDTA (pH0 10.0). Besides pH differences, coordination of phosphate species by metal cations appears to play a catalytic role in the hydrolysis mechanism via turning phosphorus atoms into suitable electrophiles for the subsequent nucleophilic attack by water. Hydrolysis rates were proportional to phosphate complex stability, with stronger complexes for chains than for rings. A competition between solvent and phosphate species for the metal ion occurred in the order EDTA > Tris > deionised water.


 Please wait while we load your content...
Please wait while we load your content...