Intercalation/deintercalation of solvated Mg2+ into/from graphite interlayers†
Abstract
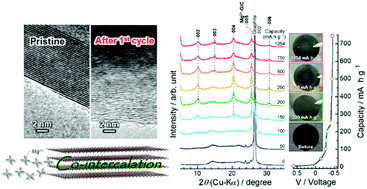
In the development of rechargeable Mg-ion batteries which are not limited by resource constraints, studies on negative electrode materials have been concentrated on efficient Mg-deposition/stripping rather than on insertion/extraction-type active materials, driven by the extremely high theoretical capacity of Mg metal (2205 mA h g−1). This work re-examined the potential of graphite, which is overlooked in electrochemical tests using a two-electrode type cell due to a large overpotential during sluggish Mg-deposition/stripping at the counter electrode caused by the passivation layer. The formation of a graphite intercalation compound (GIC) with a stage structure was demonstrated by the continual application of a constant current without considering the cut-off voltage to eliminate the detrimental impact of the counter electrode, although the intercalant was solvated Mg-ions. The GIC formed during the charging process has a blue tint just like a GIC synthesized by a vapor method. Although there is still issue with the large polarization during the deintercalation of solvated Mg ions, a reversible capacity of approximately 200 mA h g−1 could be achieved in the galvanostatic charge/discharge tests with a current density of 7.44 mA g−1. The results should facilitate future research and development of graphite as a negative electrode material.


 Please wait while we load your content...
Please wait while we load your content...