Unraveling the computed non-least motion pathway for the homodimerization of superchameleonic isocyanides: the peculiar nonsymmetrical (F–NC)2 reactant complex†
Abstract
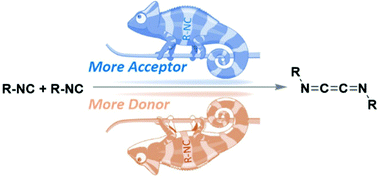
Isocyanides are commonly qualified as chameleonic compounds because of their reactions with both nucleophiles and electrophiles. In some instances, their chameleonic behavior changes to superchameleonic when they are involved in homodimerization processes, with the two initially identical isocyanide units adopting different roles along the reaction coordinate. We present here a detailed analysis of the computed non-least motion pathway that two isocyanides, the superchameleonic F–NC and, for the sake of comparison, the standard Me–NC, follow when reacting with themselves by comparing the evolution of a series of representative geometrical and electronic parameters along the respective reaction coordinates. This study shows that the two F–NC units are notoriously distinguishable from each other in all the parameters under scrutiny. Furthermore, we envisage that the superchameleonic character of F–NC seems to be most likely due to a minimal electrostatic interaction between the two entities at the earliest stage of the reaction. We also show that MeO–NC, MeS–NC and Me3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NC might be postulated as new examples of superchameleonic isocyanides.
N–NC might be postulated as new examples of superchameleonic isocyanides.


 Please wait while we load your content...
Please wait while we load your content...