Through space JFH spin–spin coupling constant transmission pathways in 2-(trifluoromethyl)thiophenol: formation of unusual stabilizing bifurcated CF⋯HS and CF⋯SH interactions†
Abstract
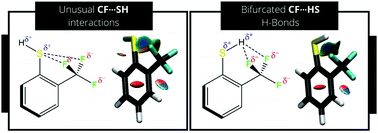
Given its importance and the possibility of organic F to participate in hydrogen bonds (H-bonds), the understanding of its behavior as a H-bond acceptor with different donors is crucial. The interest in organofluorine compounds and the works related to the study of the participation of this atom in non-covalent interactions is constantly growing. Following recent studies in this subject, we evaluated the existence of two bifurcated intramolecular interactions, a bifurcated CF⋯HS H-bond in the cis conformer of 2-trifluoromethylthiophenol and an unusual, bifurcated CF⋯SH interaction in the trans conformer. The JFH spin–spin coupling constant (SSCC) was evaluated for 2-trifluoromethylthiophenol both experimentally by 1H and 19F NMR and theoretically using the natural bond orbitals (NBO), the quantum theory of atoms in molecules (QTAIM) and the non-covalent interactions (NCI) framework. Although both interactions are crucial for the stabilization of the conformer geometries, the observed positive JFH spin–spin coupling constant (SSCC) is mainly resultant from the trans conformer, which has a large calculated positive SSCC, and is transmitted through steric interactions involving the F lone pairs and the σSH bonding orbital.


 Please wait while we load your content...
Please wait while we load your content...