Electrodeposition of neodymium and dysprosium from organic electrolytes†
Abstract
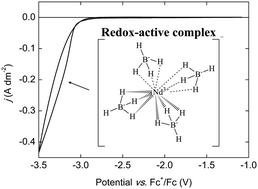
A new class of organic electrolytes has been developed for the electrodeposition of rare-earth metals at room temperature. These electrolytes consist of a rare-earth bis(trifluoromethylsulfonyl)imide or chloride salt and a borohydride salt, dissolved in the ether solvents 1,2-dimethoxyethane or 2-methyltetrahydrofuran. In these electrolytes, a soluble lanthanide(III) borohydride complex [Ln(BH4)4]− is formed, which allows for the electrodeposition of neodymium- or dysprosium-containing layers. The electrochemistry of these electrolytes was characterized by cyclic voltammetry. The deposits were characterized by scanning electron microscopy (SEM), energy-dispersive X-ray fluorescence (EDX) and X-ray photoelectron spectroscopy (XPS), and the results suggest the presence of metallic neodymium and dysprosium.


 Please wait while we load your content...
Please wait while we load your content...