Theoretical H + O3 rate coefficients from ring polymer molecular dynamics on an accurate global potential energy surface: assessing experimental uncertainties
Abstract
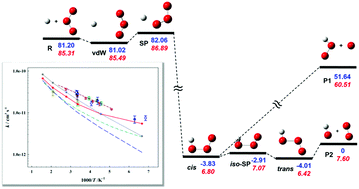
Thermal rate coefficients and kinetic isotope effects have been calculated for an important atmospheric reaction H/D + O3 → OH/OD + O2 based on an accurate permutation invariant polynomial-neural network potential energy surface, using ring polymer molecular dynamics (RPMD), quasi-classical trajectory (QCT) and variational transition-state theory (VTST) with multidimensional tunneling. The RPMD approach yielded results that are generally in better agreement with experimental rate coefficients than the VTST and QCT ones, especially at low temperatures, attributable to its capacity to capture quantum effects such as tunneling and zero-point energy. The theoretical results support one group of existing experiments over the other. In addition, rate coefficients for the D + O3 → OD + O2 reaction are also reported using the same methods, which will allow a stringent assessment of future experimental measurements, thus helping to reduce the uncertainty in the recommended rate coefficients of this reaction.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...