Properties of the tert-butyl halide solvolysis transition states†
Abstract
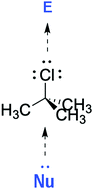
We have obtained properties (or descriptors) of the transition states in the solvolysis of tert-butyl chloride, bromide and iodide. We show that all three transition states, in both protic and in aprotic solvents, are highly dipolar and are strong hydrogen bond acids and strong hydrogen bond bases, except for the tert-butyl iodide transition state in aprotic solvents, which has a rather low hydrogen bond acidity. Thus, the transition states are stabilized by solvents that are hydrogen bond bases (nucleophiles) and are hydrogen bond acids (electrophiles). We show also that the partition of the transition states between water and solvents is aided by both nucleophilic and electrophilic solvents and conclude that the rate of solvolysis of the three halides is increased by both nucleophilic and electrophilic solvents.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...