The primary photo-dissociation dynamics of lactate in aqueous solution: decarboxylation prevents dehydroxylation†
Abstract
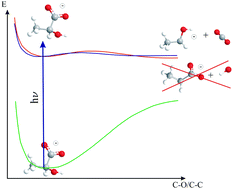
We study the primary photolysis dynamics of aqueous lactate induced by photo-excitation at λ = 200 nm. Our calculations indicate that both decarboxylation and dehydroxylation are energetically possible, but decarboxylation is favoured dynamically. UV pump – IR probe transient absorption spectroscopy shows that the photolysis is dominated by decarboxylation, whereas dehydroxylation is not observed. Analysis of the transient IR spectrum suggests that photo-dissociation of lactate primarily produces CO2 and CH3CHOH− through the lowest singlet excited state of lactate, which has a lifetime of τ = 11 ps. UV pump – VIS probe transient absorption spectroscopy of electrons from the dissociating lactate anion indicates that the anionic electron from the CO2˙− fragment is transferred to the CH3CHOH˙ counter radical during the decarboxylation process, and CO2˙− is consequently only observed as a minor photo-product. The photo-dissociation quantum yield after the full decay of the excited state is Φ(100ps) = 38 ± 5%.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...