Experimental observation of the unique solvation process along multiple solvation coordinates of photodissociated products†
Abstract
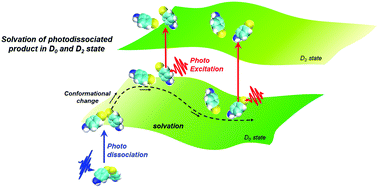
Chemical reaction dynamics in solution are closely related to solvation dynamics, and understanding solvent responses remains a crucial issue in chemistry and chemical biology. In this study, we experimentally and computationally investigated the solvation dynamics along different solvation coordinates of the same molecule: the electronically excited state and ground state of the p-aminophenylthiyl radical generated by the photodissociation of bis(p-aminophenyl)disulfide. Time profiles of the peak shifts from the transient absorption and emission spectra after photodissociation were extracted to discuss the solvent reorganization process in various ionic liquids (ILs) with different viscosities. The absorption peak position of the radical followed common solvation dynamics, shifting to a lower energy with time due to reorganization of the surrounding solvent molecules in response to the charge redistribution and molecular volume change caused by photodissociation. On the other hand, the emission band of the radical did not show a meaningful spectral shift with time. It was also found that the solvation time in the ground state was not strongly dependent on the solvent viscosity. These experimental results deviate from the conventional dynamic Stokes shift theory. To discuss the experimental results, non-equilibrium molecular dynamics simulations were conducted. The spectral shift obtained by MD simulations indicated the existence of a large solvation energy change and solvation dynamics around the radical after the photodissociation. On the other hand, the electronic excitation of the radical brought about a relatively smaller solvation energy change, especially at the long delay time after the photodissociation. These differences might be one of the reasons for the unique experimentally observed solvation dynamics.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...