Hierarchy of π-stacking determines the conformational preferences of bis-squaramates†
Abstract
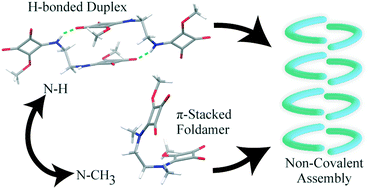
A rational design of molecular building blocks leading to their aggregation in the solid-state requires control over the intricate array of non-covalent interactions and can serve as an anchor in functional materials. Squaramides are known to form excellent hydrogen bonding and hence, can induce key functionalities in the design and synthesis of predefined patterns in crystalline solids. In the present work, a new series of mono-amides of methyl squarates (bis-squaramates 1–4) are reported, wherein the two squarates are tethered using aliphatic primary and secondary-diamine linkers of varying lengths. A simple strategy of invoking small perturbations in reported molecular structures controlled by the length and degree of substitution in the diamine linker resulted in a variety of molecular conformations and intermolecular aggregates controlled by the varying ability of H-bonding and π-stacking.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...