Stabilization of telomeric G-quadruplex by ligand binding increases susceptibility to S1 nuclease†
Abstract
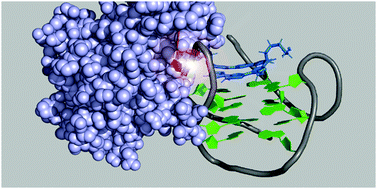
The extent of thermodynamic stabilization of telomeric G-quadruplex (G4) by isomers of G4 ligand L2H2-6OTD, a telomestatin analog, is inversely correlated with susceptibility to S1 nuclease. L2H2-6OTD facilitated the S1 nuclease activities through the base flipping in G4, unlike the conventional role of G4 ligands which inhibit the protein binding to DNA/RNA upon ligand interactions.
- This article is part of the themed collection: ChemComm Milestones – First Independent Articles


 Please wait while we load your content...
Please wait while we load your content...