Stereoselective synthesis of highly functionalized (Z)-chloroalkene dipeptide isosteres containing an α,α-disubstituted amino acid†
Abstract
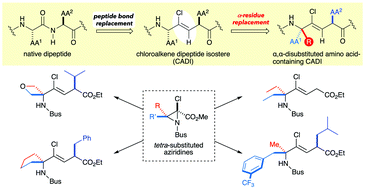
Described here is the first stereoselective synthesis of highly functionalized chloroalkene dipeptide isosteres containing an α,α-disubstituted amino acid (ααAA). This synthesis requires the construction of a quaternary carbon center, and this challenge was overcome by the Aza-Darzens condensation of ketimine with α,α-dichloroenolate, producing 2-chloroaziridines with quaternary carbon centers including spirocyclic motifs, which are valuable for the previously elusive synthesis of various ααAA-containing chloroalkene isosteres.


 Please wait while we load your content...
Please wait while we load your content...