Enantioselective synthesis of polycyclic pyrrole derivatives by iridium-catalyzed asymmetric allylic dearomatization and ring-expansive migration reactions†
Abstract
Herein, we report an N-alkylation of pyrroles triggered by an unprecedented selective ring-expansive migration of the spiro-2H-pyrrole intermediates obtained via Ir-catalyzed asymmetric allylic dearomatization. The reaction affords a series of tetrahydropyrrolo[1,2-c]pyrimidine derivatives in good yields (up to 88%) with excellent enantioselectivity (up to >99% ee). The proposed reaction mechanism is supported by DFT calculations and the characterization of the key intermediate.
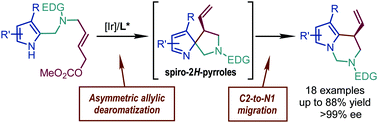
- This article is part of the themed collections: 10th Anniversary of the Youth Innovation Promotion Association of the Chinese Academy of Science and 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...