Engaging yne-allenones in tunable catalytic silane-mediated conjugate transfer reductions†
Abstract
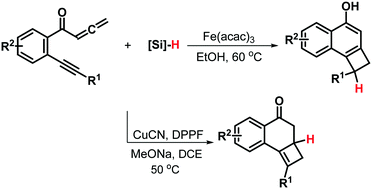
New tunable catalytic [2+2] cycloaddition/silane-mediated conjugate transfer reductions of yne-allenones have been developed, by which substituent-diverse cyclobutarenes with generally good yields were selectively synthesized by adjusting Fe–H and Cu–H catalytic systems. Use of the Fe–H system triggers 1,6-conjugate reduction to dihydrocyclobuta[a]naphthalen-4-ols whereas the Cu–H complex enables 1,4-conjugate reduction to cyclobuta[a]naphthalen-4(2H)-ones.


 Please wait while we load your content...
Please wait while we load your content...