Late-stage ligand functionalization via the Staudinger reaction using phosphine-appended 2,2′-bipyridine†
Abstract
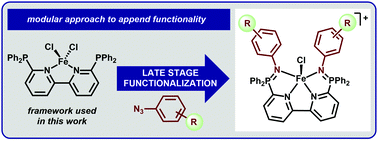
The ability of a phosphine-appended-2,2′-bipyridine ligand ((Ph2P)2bpy) to serve as a platform for late-stage ligand modifications was evaluated using tetrahedral (Ph2P)2bpyFeCl2. We employed a post-metalation Staudinger reaction to install a series of functionalized arenes, including those containing Brønsted and Lewis acidic groups. This reaction sequence represents a versatile strategy to both tune the ligand donor properties as well as directly incorporate appended functionality.


 Please wait while we load your content...
Please wait while we load your content...