Radical-mediated alkoxypolyhaloalkylation of styrenes with polyhaloalkanes and alcohols via C(sp3)–H bond cleavage†
Abstract
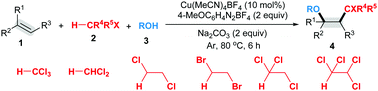
We have developed a new radical-mediated alkoxypolyhaloalkylation of styrenes with polychloroalkanes and alcohols for the facile synthesis of complex polyhaloalkanes. 4-Methoxybenzenediazonium tetrafluoroborate is a good radical initiator for this transformation. This protocol is well applied to the late-stage functionalization of complex molecules, including vitamin E, estrone and cholesterol derivatives.


 Please wait while we load your content...
Please wait while we load your content...