Benzylic C–H addition of aromatic amines to alkenes using a scandium catalyst†
Abstract
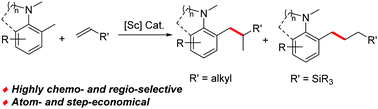
An efficient and selective benzylic C(sp3)–H addition of o-CH3-substituted tertiary aromatic amines to alkenes has been achieved using an anilido-oxazoline ligand supported scandium catalyst, which provides an atom-economic method for the synthesis of a new family of alkylated tertiary anilines. A wide range of amine and alkene substrates are compatible with the catalyst system.


 Please wait while we load your content...
Please wait while we load your content...