Transfer hydrogenations catalyzed by streptavidin-hosted secondary amine organocatalysts†
Abstract
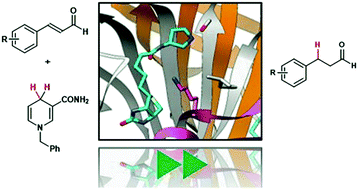
Here, the streptavidin–biotin technology was applied to enable organocatalytic transfer hydrogenation. By introducing a biotin-tethered pyrrolidine (1) to the tetrameric streptavidin (T-Sav), the resulting hybrid catalyst was able to mediate hydride transfer from dihydro-benzylnicotinamide (BNAH) to α,β-unsaturated aldehydes. Hydrogenation of cinnamaldehyde and some of its aryl-substituted analogues was found to be nearly quantitative. Kinetic measurements revealed that the T-Sav:1 assembly possesses enzyme-like behavior, whereas isotope effect analysis, performed by QM/MM simulations, illustrated that the step of hydride transfer is at least partially rate-limiting. These results have proven the concept that T-Sav can be used to host secondary amine-catalyzed transfer hydrogenations.


 Please wait while we load your content...
Please wait while we load your content...