Iodine-promoted ring-opening methylation of benzothiazoles with dimethyl sulfite†
Abstract
A halogen-bond promoted ring-opening methylation of benzothiazoles has been developed using dimethyl sulphite as a methylating reagent in the presence of a base. This approach represents a simple and efficient synthesis of N-methyl-N-(o-methylthio)phenyl amides, and features direct construction of both N–Me and S–Me bonds in a one-pot reaction through the decomposition of easily prepared benzothiazoles.
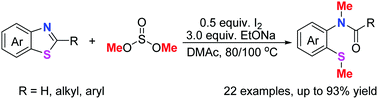


 Please wait while we load your content...
Please wait while we load your content...